Abstract
Background: There are limited real-world data (RWD) available on the unmet needs of people with mild or moderate hemophilia A (PwHA). This population accounts for 40-52% of all PwHA, including nearly all women with hemophilia A (HA), and is under-represented in scientific literature (Michele, et al. Haemophilia 2014; Benson, et al. Blood Transfus 2018; Peyvandi, et al. Haemophilia 2019). Available claims data from payer databases are confined to billing codes, and lack key information on outcomes and disease characterization (e.g. severity, treatment response.) (Tyree, et al. Am J Med Qual 2006). Registry datasets can require resource-intensive data entry and potentially miss key information about care received at outside facilities, at home, or after patients switch providers (Gliklich, et al. Registries for Evaluating Patient Outcomes: A User's Guide. 2014). To address these data gaps, we developed a novel, patient-centered approach to create a longitudinal healthcare database from individuals with mild and moderate HA in the United States. This study assessed the feasibility of this approach, which integrates medical record data collected during routine clinical care along with patient-reported outcomes (PROs) to provide needed insights into this under-represented population.
Methods: Recruitment began in June 2020 via a broad strategy of social media outreach, healthcare provider partnerships, and patient advocacy groups. Eligibility was confined to mild or moderate PwHA, confirmed via physician report within provider notes in combination with baseline factor VIII levels (>5-50% mild, 1-5% moderate.) This study received research ethics board approval and abides by the guiding principles of the Declaration of Helsinki.
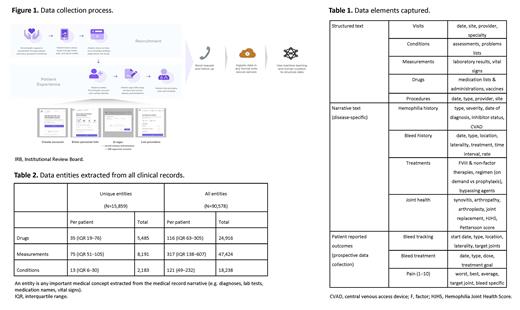
PwHA enrolled via an online record management platform, PicnicHealth. After signing authorization forms for collection of their electronic health records (EHR) and informed consent to share their de-identified data for research, participants were prompted to enter information on their care providers. Records were gathered from all providers, across any facility, retrospectively as records were available. (Figure 1) All records obtained were made available to the participants via a medical timeline.
Records were translated to text via optical character recognition with human review. Data elements from structured text as well as disease-specific elements from narrative text were captured using natural language processing and supervised machine learning. All elements, including visit metadata, conditions, measurements, drugs, and procedures were mapped to standardized medical ontologies and reviewed by a team of nurses. (Table 1) Quality control was assessed via inter-abstractor agreement on outputs with physician review.
Patient-reported bleed, treatment, and pain data were collected via online questionnaire for a subset of PwHA, with participants prompted to enter data every 2 weeks. Abstracted EHR data was linked to PRO responses in a de-identified dataset. Cohort and abstraction characteristics were summarized descriptively.
Results: From June 1, 2020 to June 30, 2021, 104 PwHA met eligibility criteria for enrollment (65 [62.5%] mild; 39 [37.5%] moderate). Participants saw providers across 34 states in the US, 22.1% (23/104) were female, and 20.6% (14/68) of those with known race/ethnicity status were from minority groups.
Records were gathered from a median of six care sites and 16 providers per participant. A median of 50 (IQR [21-93]) clinical documents from 11 years were processed for each PwHA. (Table 2) Inter-abstractor agreement to assess abstraction quality averaged 95.9% for condition, 99.5% for drug name, and 95.4% for drug start date. As of June 2021, the average PRO response rate was 90.3% (150/166 of all requests) and continues prospectively.
Conclusions: The patient-centric data collection methods implemented in this study provide a novel approach to build longitudinal real-world data sets. Technology-enabled data abstraction showed consistent high quality when processing the heterogeneous clinical records across disparate providers and care sites, and direct engagement with patients complements potential gaps in the clinical record. Additionally, this approach provides needed data on groups under-represented in RWD and traditional PwHA cohorts, including those with mild and moderate disease and women with HA.
Skinner: ICER: Membership on an entity's Board of Directors or advisory committees; Spark (DMC): Honoraria; Sanofi: Honoraria; F. Hoffmann-La Roche Ltd/Genentech, Inc.: Honoraria; Pfizer (DMC): Honoraria; Bayer: Honoraria, Membership on an entity's Board of Directors or advisory committees; uniQure: Research Funding; Takeda: Honoraria, Research Funding; F. Hoffmann-La Roche Ltd: Research Funding; Freeline: Research Funding; BioMarin: Honoraria, Research Funding; IPA Ltd.: Current holder of individual stocks in a privately-held company; National Hemophilia Foundation: Consultancy; Institute for Policy Advancement Ltd: Current Employment; WFH USA: Membership on an entity's Board of Directors or advisory committees; BCBS MAP: Membership on an entity's Board of Directors or advisory committees. Hanson: PicnicHealth: Current Employment, Current holder of stock options in a privately-held company. Xu: F. Hoffmann-La Roche AG: Current Employment. Ofori-Asenso: F. Hoffmann-La Roche Ltd: Current Employment. Ko: Genentech, Inc.: Current Employment; Genentech, Inc.-Roche: Current equity holder in publicly-traded company, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company. Cibelli: PicnicHealth: Current Employment. Nissen: Novartis: Consultancy; Actelion: Consultancy; F. Hoffmann-La Roche Ltd: Current Employment, Current holder of stock options in a privately-held company. Witkop: Roche Advisory Panel: Consultancy; National Hemophilia Foundation: Current Employment. Sanabria: F. Hoffmann-La Roche Ltd: Current Employment, Current holder of individual stocks in a privately-held company. Shapiro: Novartis: Research Funding; Novo Nordisk: Other: Advisory board fees, Research Funding, Speakers Bureau; Octapharma: Research Funding; Pfizer: Research Funding; OPKO: Research Funding; Prometric BioTherapeutics: Research Funding; Sangamo: Other: Advisory board fees, Research Funding; Sigilon Therapeutics: Other: Advisory board fees, Research Funding; Takeda: Research Funding; Kedrion Biopharma: Research Funding; Glover Blood Therapeutics: Research Funding; Genentech: Other: Advisory board fees, Research Funding, Speakers Bureau; Daiichi Sankyo: Research Funding; Bioverativ (a Sanofi company): Other: Advisory board fees, Research Funding; BioMarin: Research Funding; Agios: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal